Coaggregation: Interactions between Bacteria in Dental Biofilms
Student Version (go to Instructor Version)
CONTENT
In 1970, Gibbons and Nygaard observed that when pure cultures of some dental plaque bacterial strains were mixed, the suspension rapidly cleared. For example, when broth cultures of Actinomyces naeslundii and certain strains of Streptococcus sanquinis were combined a marked decrease in turbidity occurred within minutes1. This phenomenon, now termed coaggregation, is of selective value to bacterial living in a flowing environment as any cells, which detach from the oral surface are washed away and swallowed. Kolenbrander and others working on this phenomenon have found that coaggregation is exhibited by nearly all oral bacteria tested, a sample which includes more than seven hundred bacterial strains representing at least 18 genera2,3,4,5.
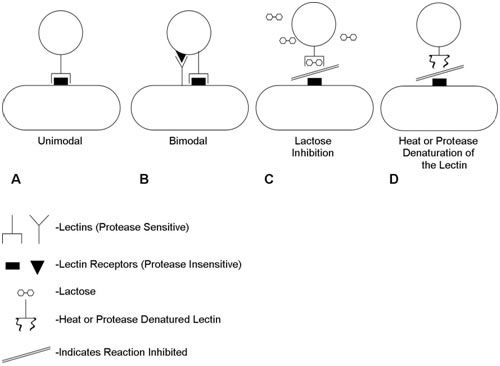
It is now known that the dominant cell-cell interactions are between Lectin type (protein) adhesins on one of the cells and oligosaccharide moieties on the other. Coaggregation can be interrupted by denaturation of the lectin or, frequently, by the addition of sugar (e.g. lactose) which blocks the active lectin site (Figure 1). Figure 1 shows the nature of the interactions between coaggregating pairs of organisms. Some pairs have just a single known lectin/receptor pair (unimodal) while others may have two (bimodal). Coaggregation may be inhibited either by denaturation of the protein lectin or by competitive inhibition of the lactin/cagbohydrate interaction by a sugar (e.g. lactose).
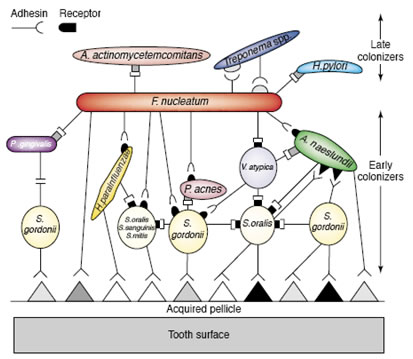
This array of coaggregation associations is related to the succession by which dental plaque matures. Early colonizers predominantly Streptococci attach to a conditioning film. Subsequently, actinomycetes and fusobacteria bind by coaggregation to these pioneer species. Depending largely upon the degree of dental hygiene, other organisms join the biofilm. These late arriving species have little ability to coaggregate with the pioneers, but do bind with the intermediates such as Fusobacterium. The fusobacteria are therefore called bridging species.
Figure 2 shows a diagrammatic sketch of the arrangement of bacterial strains which form dental biofilms. An acquired pellicle or conditioning film composed of proteins drawn from the saliva or crevicular fluid first attaches to the tooth surface. Pioneering bacteria, largely streptococci, attach to these proteins. Other organisms attach to these pioneers largely by means of coaggregation between a protein lectin on one organisms and a carbohydrate receptor on the other From Rickert et al. 2003, used with permission.
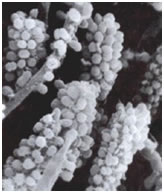
Among the most interesting morphological structures observable in samples of oral biofilms are the so-called “corncobs” or “test tube brush formations”, by the investigators who first described them (Figure 3). In these structures cocci coaggregate with rod-shaped or filamentous bacteria producing the characteristic corncob shape. These can occasionally be seen in negative stained preparations of dental tartar or plaque viewed under the brightfield microscope. An analogous formation called a “rosette” consists of a single coccus-shaped cell of one bacterial strain coaggregating with a larger number of cocci of a different type completely surrounding the first.
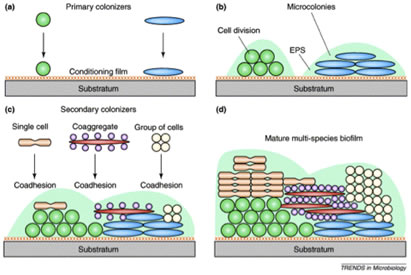
Dental biofilms “grow” by two mechanisms, accretion in which additional microorganisms are added successively to the community, increasing its diversity and cell division of the individual cells forming microcolonial islands which contribute to the biofilms mass (Figure 4).

Specific adhesins on one member of a pair of organisms may bind to cognate receptors on the other forming masses of bacteria the size of which depends on the strength of the coaggregation reaction. These masses form very rapidly and can be easily viewed with the naked eye, although back-lighting and some magnification makes quantification much easier.
Figure 5 shows the results of a coaggregation assay. Tube one (on the left) is a pure culture showing no coaggregation, tube 2 (center tube) indicates a strong +4 reaction, while tube 3 (on the right) should be read as a +2 reaction.
In this exercise you will explore the nature and strength of coaggregation between pairs of oral bacteria and learn how to determine which of the bacterial partners bears the protein lectin component and which the carbohydrate. You may also observe the roll of certain sugars in inhibiting the lectin/carbohydrate interactions.
You may also explore the nature and strength of coaggregation between pairs of oral bacteria and learn how to determine which of the bacterial partners bears the protein lectin component and which the carbohydrate. You may also observe the roll of certain sugars in inhibiting the lectin/carbohydrate interactions.
MATERIALS AND EQUIPMENT
| Per student or student group |
Item |
|---|---|
| 20 - 30 | 10 X 75mm or 12 X 75mm glass test tubes |
| 10 ml | Each of the reference strains in Table 1 |
| 4 ml | 300 mM lactose |
| 1 each | 200 μl, 20 μl, and 1000 μl pipettor with appropriate tips |
| Per Lab | Item |
|---|---|
| 1 | 85° waterbath or dry heat block |
ORAL BACTERIAL REFERENCE STRAINS
| Bacterium | Strain | Coaggregation Group |
|---|---|---|
| Actinomyces naeslundii | PK 35 | A |
| Streptococcus gordonii | ATCC 35105 | 1 |
| Streptococcus gordonii | ATCC 51656 | 6 |
| Streptococcus oralis | 34 | 3 |
| Streptococcus oralis | J22 | 4 |
Student Notes:
-
Look over the list of materials and bacterial strains. These materials and pieces of equipment should all be available in the laboratory. The bacterial strains have been grown in nutrient medium, concentrated by centrifugation and resuspended in coaggregation buffer. The strains used in this exercise are all BSL 2 (Biosafety Level 2) organisms which means they can be handled at the bench without special techniques or apparatus, but remember all microorganisms should be treated with respect and due caution. For a description of Biosafety Levels, see http://en.wikipedia.org/wiki/Biosafety_level.
-
Consult your instructor about disinfectant and barrier protection procedures (gloves, aprons).
-
An aerosol is a suspension of bacterial cells in air. In this form, many bacteria are considered to be easily transmitted to the respiratory tract of laboratory workers and to surfaces in the laboratory. Often it will not even be evident that aerosolization has occurred. During these exercises you will be mixing bacteria in test tubes on a Vortex® Mixer. This is an operation that may produce aerosols. Your instructor will give you instructions to reduce the probability of forming aerosols
DETAILED PROCEDURES
Exercise 1: Measurement of auto and coaggregation.
You are provided with the materials described in Tables 1 and 2 above.
- Each individual strain should be tested for autoaggregation (control) by placing 400 μl of the bacterium in a small test tube. The tube is vortex mixed for 5 seconds and then rocked back and forth while observing aggregation formation. Any degree of settling or clearing of the tube indicates autoaggregation and this number (from
assessment scale, see Table 3) should be subtracted from the value for coaggregation.
Table 3. Quantification of Coaggregation Observation Coaggregation Score Description 0 Evenly turbid suspension of bacteria 1 Finely dispersed clumps in a turbid background 2 Definite clumps of bacteria are easily seen but do not settle immediately and remain in a turbid background 3 Clumps settle immediately with a slight turbid background 4 Clumps settle immediately and the supernatant is completely clear
Good lighting is essential for viewing the extent of coaggregation. We have found that the surface of an overhead projector works well and a Quebec Colony Counter with back lighting ad magnification works even better (Figure 6).
 Figure 6. Quebec Colony Counter
Figure 6. Quebec Colony Counter
- Pairs of organisms are tested for coaggregation by mixing together, 200 μl of each strain in a test tube. The tube is vortex mixed (~5 seconds) and then rocked back and forth until aggregates form.
Score each assayed pair. An easy way to keep track of each coaggregation assay is to set up a simple table with each strain represented on each axis (see Kolenbrander et al. 1990, or Clemans and Kolenbrander, 1995 for examples).
Score each assay 0 - 4 using the scale indicated above for the initial coaggregation score.
- For the laboratory report, identify any pairs of reference strains that exhibit coaggregation and produce a table of your results.
Exercise 2: Reversal of coaggregation with Lactose
- Many Lectin carbohydrate interactions may be reversed by competitive inhibition with sugars such as lactose.
- Select each of the coaggregation pairs prepared in Exercise 1 that showed any sign of coaggregation. To each add 100 μl of a 300 mM stock solution of lactose. This produces a final concentration of 60 mM lactose.
- Once again vortex mix the tubes for 5 seconds and rock the tubes while observing for coaggregation.
Note: If desired, other sugars can be added instead to a final concentration of 15 mM 6. - An easy way to keep track of each coaggregation assay is to set up a simple table with each strain represented on each axis (see references 6 or 7 for examples). Indicate the coaggregation score with lactose as a superscript to the original score without lactose (e.g. 40, 31 ).
- Do you observe any difference in the coaggregating pairs with and without Lactose? For the Laboratory report add this new information to your table from Section 1.
Exercise 3: Assessment of the Nature of the Interactions Between Bacterial Strains
- To determine which partner contains the protein adhesin, incubate 2 ml of each bacterium at 85°C for 30 min, and then perform coaggregation as directed above.
Compare these results with the results in exercise 1. - Those bacterial strains that lose the ability to coaggregate with their partners, after heat treatment contain the protein adhesin. Those treated bacterial strains that still coaggregate with their partners contain the carbohydrate receptor.
- Each bacterial strain may possess an adhesin, a receptor or both (Figures 2 and 6).
- The following table may be useful in setting up the coaggregation pairs between heated and non-heated strains. A and B indicate the members of a pair, the H indicates that that member of the pair has been heated.
| Assay | Interpretation |
|---|---|
| A x B | As in Section 1 |
| AH x B | A lowering of the Coaggregation score implies that A possesses a Lectin. |
| A x BH | A lowering of the Coaggregation score implies that B possess a Lectin |
| AH x BH | Further lowering of the Coaggregation score beyond either of the above trials implies that both strains possess Lectins (Bimodal Coaggregation) |
FOLLOW-UP ACTIVITIES
- Coaggregation has been studied most intensively in dental plaque and to a lesser extent in fresh water environments. The field of investigation is wide open to explore coaggregation in any other natural (or human influenced) ecosystem. Organisms isolated from soil, sink drains, shower curtains, and any other habitat are potential materials for investigations of coaggregation.
- Exploration of bacterial coaggregation occurring in laboratory teaching strains of streptococci and other bacteria. Many teaching laboratories use stock strains for teaching purposes. The coaggregation properties of these strains, especially streptococci, can be assessed using the procedure followed in Section I.
- Culture the bacterial strain in an appropriate growth medium and wash the bacterial cells in coaggregation buffer as indicated above. Resuspend the bacterial suspension to a final cell density of A600 1.0 (~109 bacterial cells/ml).
- Assess the coaggregation properties as in Sections 2 and 3. Determine which partners contain the protein adhesin, and which strains contain the carbohydrate receptor.
QUESTIONS
1. Which pairs of oral bacterial strains are inhibited by Lactose? What is the significance of this observation?
2. Which organism in each case bears the lectin adhesin?
3. In an evolutionary sense, what selective advantage do bacteria capable of coadhesion and coaggregation have over bacteria not capable of coaggregation?
REFERENCES:
- Gibbons, R. J. and M. Nygaard, 1970, Interbacterial Aggregation of Plaque Bacteria. Archives of Oral Biology 15:1397-1400.
- Kolenbrander, P.E., and R.J. Palmer Jr., 2004 Human Oral Bacterial Biofilms, In M. Ghannoum and G.A.O’Toole, Microbial Biofilms. ASM Press, Washington D.C.
- Rickard, A.H. P. Gilbert, N.J. High, P.E. Kolenbrander, and P.S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends in Microbiology 11:94-100.
- Kolenbrander, P.E., R.N. Andersen, D.S. Blehert, P.G. Egland, J.S. Foster, and R.J. Palmer Jr. 2002. Communication among oral bacteria. Microbiol. Molec. Biol. Rev. 66:486-505.
- Kolenbrander, P.E. 1989. Surface recognition among oral bacteria: multigeneric coaggregation and their mediators. Crit. Rev. Microbiol. 17:137-159.
- Kolenbrander, P.E., R.N. Andersen, and L.V. Moore. 1990. Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl. Environ. Microbiol. 56:3890-3894.
- Clemans, D.L., and P.E. Kolenbrander. 1995. Isolation and characterization of coaggregation-defective (Cog-) mutants of Streptococcus gordonii DL1 (Challis). J. Industrial Microbiol. 15:193-197.
- Kolenbrander, P.E. 1988. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Ann. Rev. Microbiol. 42:627-656.
Developed in collaboration with Dr. John Lennox, Penn State Altoona and Daniel L. Clemans, Eastern Michigan University . Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. ©2002-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
