Biofilms as Biobarriers
Instructor Version (go to Student Version)
| Subject Area(s) | microbiology |
| Intended Audience |
high school biology, independent study/science fair, introductory undergraduate microbiology, advanced college level microbiology |
| Type | laboratory exercise |
| Revision Date | May 25, 2007 |
CONTENT
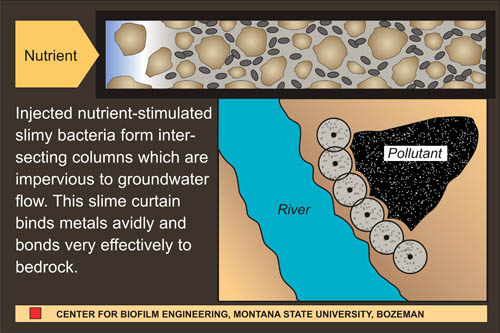
One of the defining characteristics of biofilms is the production of a slimy matrix made of polysaccharide, protein and sometimes DNA. This extracellular polymeric substance (EPS) has often been considered a nuisance, particularly when it accumulates on the hulls of ships, in industrial pipes or on teeth. More recently, microbiologists and engineers have been looking at biofilms and their EPS production as a means of solving serious environmental pollution problems. When injected into the soil and fed an appropriate diet, organisms will grow and plug the interstices between soil particles with EPS. If drill holes are sited in advance of a contaminated ground water plume, this “biobarrier” can significantly reduce the rate of flow (See Figure 1).1,6
PREREQUISITES
Students should be able to define a biofilm, describe the differences between biofilm (surface-attached) and planktonic (free-floating) bacteria, and be able to describe the growth of bacteria on surfaces. Students should also be familiar with standard methods handling and transferring microbial cells with aseptic technique. Students should also have some basic understanding of the composition of soil, its structure and of ground water movement through soil.INSTRUCTIONAL OBJECTIVE
Given a glass column packed with sand, an overnight culture of a BSL-1 organism (e.g. Pseudomonas fluorescens, P. putida, Bacillus subtilus or B. cereus) and detailed instructions, the student will be able to measure the effect of biofilm formation on “ground water” flow. Students should be able to explain the use of biofilms in containing the deleterious effects of ground water pollution.INSTRUCTIONAL PROCEDURES
Materials and Methods
The demonstration of biobarrier technology outlined in this poster employs a column partially packed with tiny glass beads or sand. A significant space (head space) is left above the packing for medium so that the rate of flow through the column can be measured. The column is inoculated with a microorganism and fed a medium formulated to produce large volumes of EPS. At intervals the column is filled with a measured volume of fresh medium, and the time required for the medium to drain from the column is measured. The rate of flow (ml/sec) is a measure of the efficiency with which the flow of medium is being retarded. The effects on flow rate of various media, sand pore size, BSL-1 organism, temperature etc. can be measured by different student groups. Alternatively, flow rate measured by the entire class in multiple trials can serve as the basis for statistical analysis.Notes on Safety: Latex or other gloves are highly recommended for all procedures involving adding culture or medium to the column and in measuring flow rate. It is strongly suggested to use only BSL 1 organisms for this exercise. As a precaution and to guard against “catastrophic failure” of the column or spillage, it is advisable to place the entire apparatus in a pan or tray capable of containing the entire volume of the column and waste disposal beaker. We have not had such a failure but consider this precaution prudent. Students and technicians should wear hand and eye protection during the cutting of glass tubing and the fitting of this tubing into rubber stoppers.
Detailed instructions:
In an advanced class you might have students assemble the columns themselves. If used in an introductory class it might be best to assemble the columns ahead of time. This is a complex preparation but once the columns are made they are reusable.
- From 1 cm flint glass tubing stock, cut a section of glass tubing to form a column approximately 10 - 12 inches (25 - 30 cm) long. Grind or fire-polish the column ends. Into each end of the column, fit a Number 00 one-hole rubber stopper. Each stopper should have a glass or autoclavable plastic tube extending beyond the end of the stopper (see Figures 2 and 3).
- Column top: During draining of the tube, sterile air must enter the top of the column to maintain the tube as a monoculture. To allow sterile air to enter the column, fit a bacterial air vent (0.2 µm pore size, e.g. Pall Life Sciences # 4210) to the rubber stopper at the top of the column using a Tygon™ tubing sleeve (Figures 2 and 3). As an alternative to using the air vent, extend the length of the glass tube and lightly pack it with roll cotton. After autoclaving, this will act as a bacteriological filter.
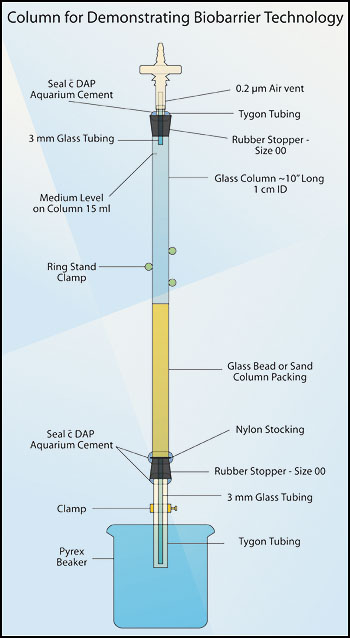 Figure 2. Column for demonstrating biobarrier technology.
Figure 2. Column for demonstrating biobarrier technology.
- Using aquarium cement (e.g. DAP™), seal the Tygon™ tubing to the rubber stopper. After this cement has dried (about 24 hours), it is autoclavable.
- Column base: When inserting the lower rubber stopper, place a square of nylon stocking between the column and the rubber stopper. Trim the nylon as close to the column as possible. This barrier holds the packing material in the column and out of the delivery tube. Attach an 8 cm length of Tygon™ tubing to the glass tubing extending from the bottom stopper, and tighten a screw clamp on this tubing (see Figures 2 and 3).
- Using a double thickness of aquarium cement, seal the bottom stopper to the column, and seal the Tygon™ tubing to the glass tubing (see Figure 2).
- Pack the lower 8 cm of the column with glass beads or washed builders sand. The smaller the particles the more quickly one will see the biobarrier effect. Close the clamp and add just enough water to fill the column to the surface of the packing material.
- Add 15 ml of water to the column and mark the 15 ml level with a permanent marker or piece of autoclavable tape. Then drain the column.
- Cover the lower delivery tube and the top of the column (including the filter) with aluminum foil and autoclave.
- Fix the sterile column securely in a clamp attached to a ring stand.
- Any bacterium that produces abundant EPS will serve as a biobarrier agent, but for this application we recommend using only BSL-1 agents. The organism used in this study was Pseudomonas fluorescens. Grow the organism up in an overnight culture in molasses medium (see formula below), on a shaker at room temperature. Note: Many “domesticated” strains grown through hundreds of passes in the laboratory, loose much of their ability to form biofilm. If you are unsure if your culture produces sufficient “slime,” grow it up on an agar plate. Touch the top of a colony with a sterile loop and lift. The presence of a slimy stringy mass indicates that the culture is a likely candidate.
- Add enough of the culture to the top of the column to bring the level to the 15 ml line previously marked. Remove the clamp while holding the Tygon™ tubing closed. Release the Tygon™ tubing, and with a stopwatch record the time taken for the culture to reach the surface of the packing material. Record this value as the pre-biofilm drainage time. Re-attach the clamp and incubate the column at room temperature until the next laboratory period. Make sure the medium is just at or slightly above the packing material surface.
- Continue to take readings on the flow rate of the column as long as any significant change is noted. Class data can be pooled and the results placed in a graph.

The culture:
Lab period one:
Lab period two: 12. Add molasses medium to the column up to the 15 ml mark. As before, remove the clamp while holding the Tygon™ tubing closed. Release the tubing and record the time taken for the medium to just reach the surface of the packing material. We find it convenient to provide the molasses medium in individual 20 ml aliquots in capped culture tubes.
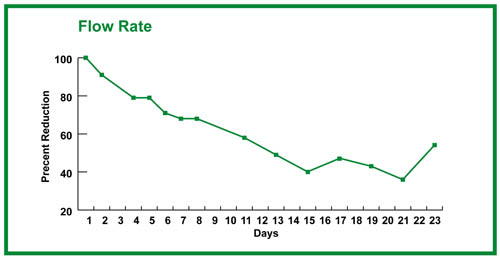
With growth, one should see the drainage time lengthening. This can be recorded in seconds, but recording ml/sec may be a more useful measure of biobarrier formation (15 ml/drainage time in sec = ml/sec; see Figure 4).
Subsequent lab periods:
Molasses Medium Formula4
Quantity |
Description |
| 10g/l | molasses |
| 0.123 g/l | K2HPO4 |
| 0.04 g/l | KH2PO4 |
| 1 g/l | NaCl |
| 3 g/l | NaNO3 |
| 2 g/l | NH4NO3 |
| 0.005 g/l | yeast extract |
MATERIALS AND EQUIPMENT
Quantity |
Description |
| 1 | Glass column packed with sand (see Figures 2 and 3) |
| As Necessary | Sterile molasses medium (20 ml aliquots) in capped culture tubes |
| As Necessary | An overnight culture of Pseudomonas fluorescens (or other BSL-1 organism) grown in molasses medium |
| As Necessary | Sterile pipettes, 25 ml size |
| 1 | 150 ml beaker |
| 1 | Stopwatch |
| 1 | Container for the disposal of waste medium |
| As Necessary | Gloves |
ASSESSMENT / EVALUATION
The instructor may assess the exercise by comparing flow rate reduction curves of individuals or groups of students. Flow rate will depend on many factors including pore size of the column packing material (smaller pore size yields more rapid response), the EPS production of the culture, the medium and temperature.FOLLOW-UP ACTIVITIES
If there exists an organism capable of metabolizing the contaminating material, the biobarrier may not only slow the spread of the contaminant but may also result in its mineralization (conversion to inorganic byproducts). This is called a reactive biobarrier and this strategy has been demonstrated repeatedly in laboratory and field tests and occasionally in full scale attempts to reduce the impact of significant pollution events. This was done, perhaps most notably, in the successful attempt to reduce the ground water levels of MTBE (a gasoline additive) that leaked from a underground storage tank on the naval base at Port Huenema, CA.2,3,In this exercise, the column is “fed” molasses medium containing nitrate (see medium). Nitrate is a common contaminant in US ground water supplies. The disappearance of nitrate ion, or the appearance of nitrite ion in the column effluent indicates the action of the P. fluorescens as a reactive biobarrier agent.5 The presence of nitrate or nitrite in water can be determined by using the appropriate LaMotte test kit available from suppliers of scientific equipment.
REFERENCES
1Subsurface biofilm barriers for the containment and remediation of contaminated groundwater
Cunningham AB, Sharp RR, Hiebert R, James G
Bioremediation Journal, 2003; 7:151-164
2In situ bioremediation of MTBE in groundwater
Johnson PC, Bruce CL, Miller KD
Technical Report TR-2222-Env. 2003. Naval Facilities Engineering Service Center, Port Huenema, CA ESTCP Project No. CU-0013
3In situ bioremediation of MTBE using biobarriers of single or mixed cultures
Salanitro JP, Spinnler GE, Maner PM, Tharpe DL, Pickle DW, Wisniewski HL
in A Leeson, BC Alleman, PJ Alvarez and VS Magar (eds.) Bioaugmentation, Biobarriers and Biogeochemistry, Battelle Press, Columbus, OH, 2001.
4Biofilm barrier formation and persistence in variable saturated zones
Komlos J, Cunningham AB, Warwood B, James G
In Proceedings of the 1998 Conference on Hazardous Waste Research, pp 200-208.
5Prokaryotic nitrate reduction: Molecular properties and functional distinction among bacterial nitrate reductases
Moreno-Vivián C, Cabello P, Martínez-Luque M, Blasco R, and Castillo F
Journal of Bacteriology, 1999; 181:6573-6584
6Biofilms in porous media
Cunningham AB, Bouwer EJ, Characklis WG
in WG Characklis and KC Marshall (eds.) Biofilms, John Wiley & Sons, Inc., New York, 1990, pp 697-732.
Educational Program Curricula and Teaching Resources
Supported in part by the Waksman Foundation for Microbiology
Developed in collaboration with Dr. John Lennox, Education Editor, Penn State Altoona
© 1999-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
