Construction Project |
Collecting Biofilms |
Biofilm Growth |
Visualizing Biofilms |
Quantification |
Harvesting Cells |
Quorum Sensing |
Environmental Application |
Industrial Application |
Clinical Application |
Classroom Exercises |
| Exercise 1 - Building and Using a Batch Biofilm Growth Reactor (Mason Jar Reactor) description - instructor version - student version |
X | X | X | X | X |
|---|
Type: Con., Grow, Vis., Quant., Harv.
Description: This exercise gives detailed instructions for building an inexpensive biofilm batch reactor from readily available materials.
Application: This batch reactor permits the student to grow a biofilm on a 1 x 3 inch glass microscope slide with shear forces created by a magnetic stir bar. In a batch reactor the culture goes through the standard growth curve stages (Lag, Log, Stationary and Death). Culture conditions change continuously as nutrients are exhausted and wastes accumulate. Up to 4 biofilm coated slides can be "grown" simultaneously. It can be used with either pure or mixed cultures.
Connectivity: This exercise can be linked to labs, which Visualize the Biofilm (Ex. 16), Measure its Thickness (Ex. 9), and Harvest, Disperse and Enumerate cells (Exs. 6,7,8).
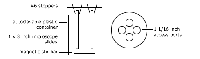
Biofilm Batch Reactor
close description
| Exercise 2 - Building a Biofilm Model from a Confocal Microscope Stack description - instructor version - student version |
X | X | X |
|---|
Type: Con, Vis, Class
Description: Images taken by a Confocal Scanning Laser Microscope are of limited depth of field and are stored individually in the memory of a computer like so many individual slices of bread. The computer can reassemble these "slices" to produce a three dimensional "whole loaf". Paul Stoodly has provided a "confocal stack" from which individual students or classes can "cut out" and reassemble their own model of a biofilm. Once assembled the students can answer questions about the original such as direction of flow, overall dimensions, architecture and the presence of water channels.
Application: For more visual and tactile learners this exercise permits the construction and visualization of a biofilm model taken from "life". Connectivity: This exercise can be used as a "hands on" introduction to other exercises (e.g. Exs. 16 and 21) in which they will observe "real" biofilms.

close description
| Exercise 3 -Building and Using a Biofilm Continuous Flow Stirred Reactor description - instructor version - student version |
X | X | X | X | X |
|---|
Type: Con, Grow, Vis, Quant, Harv
Description: This exercise describes the construction of a Continuous Flow Stirred Reactor from readily available and inexpensive materials. While similar to the Batch reactor described in Ex. 1 this device is more like a chemostat than a culture tube. Nutrients are constantly fed so that the culture growth phase can be controlled. Once established, conditions such as nutrient concentration and waste product levels remain more or less constant. If the flow rate is greater than the growth rate, only cells growing on the 1 x 3 microscope slide and the walls of the CFSR remain in the reactor.
Application: This CFSR reactor permits the student to grow a biofilm on a 1 x 3 inch glass microscope slide with shear forces created by a magnetic stir bar. Up to 4 biofilm coated slides can be "grown" simultaneously. It can be used with either pure or mixed cultures. Connectivity: This exercise can be linked to labs, which Visualize the Biofilm (Ex. 16), Measure its Thickness (Ex. 9), and Harvest, Disperse and Enumerate cells (Exs. 6,7,8).
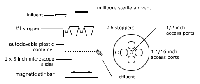
close description
| Exercise 4 -Building and Using a Flow Cell Biofilm Reactor description - instructor version - student version |
X | X | X |
|---|
Type: Con, Grow, Vis
Description: A Flow Cell is a type of biofilm reactor, which permits the continuous microscopic observation of a developing biofilm from irreversible attachment to maturity and dispersal. It is a type of Plug Flow reactor in which conditions at any point in the cell remain relatively constant although conditions in other part of the cell may be quite different. The flow cell described can be built of easily obtained materials and provides one of the most dramatic real time illustrations of biofilm structure and growth obtainable.
Application: Observations can be made of biofilm growth and development in real time over an extended period. Students can view the viscoelacitic properties of biofilms with bulk fluid flow and often see dramatic sloughing events as large portions of the biofilm break off. Connectivity: Can be used in coordination with model building (Ex. 2). If an access port is included in the construction, additional organisms, nutrients and stains can be added during operation.

close description
| Exercise 5 - Collecting Soil Biofilms by the Buried Slide Technique description - instructor version - student version |
X | X | X | X | X | X |
|---|
Type: Coll, Grow,Vis, Quant, Harv, Env
Description: This useful technique permits students to grow soil biofilms on a 1 x 3 glass slide.
Application: The biofilms collected by this technique may be harvested and counted, or stained for visualization.
Connectivity: This exercise may be linked to Ex. 8, the flow through Gram stain technique. The slide produced in this exercise can be used as a source of cells for harvesting (Exs. 9 and 10) and for Enumeration by Drop Plate Counting (Ex. 7). One might also try to measure the thickness of the biofilm (ex. 12).
close description
| Exercise 6 - Colorimetric Measurement of Biofilm Density description - instructor version - student version |
X | X | X |
|---|
Type: Grow, Vis, Quant
Description: This exercise employs the fact that the extracellular polysaccharide matrix of a biofilm stains densely with dyes like crystal violet (CV) and safranin. Biofilms are grown in a 24 well plate (or 96 well if you have a plate reader). The wells are washed and then stained with CV. The wells are then washed to remove unbound CV and then the CV bound to the biofilm matrix is eluted and measured in a spectrophotometer. With a little practice it is dramatic and reproducible.
Application: This technique is widely used in 1) isolating biofilm deficient mutants, 2) measuring growth of a biofilm over time, 3) measuring biofilm forming capability of natural isolates and many other projects.
Connectivity: This exercise can be used in any application where one wishes to measure the amount of biofilm growth. For example if students have pure culture isolates from a native biofilm and wish to know which are the greatest contributors to EPS production, this would be a great choice.
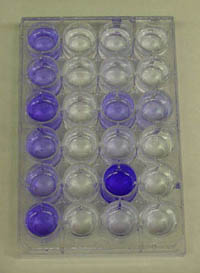
close description
| Exercise 7 - Drop Plate Method for Counting Biofilm Cells description - instructor version - student version |
X | X |
|---|
Type: Quant, Harv
Description: The Drop Plate method was developed to make it possible to do serial dilutions on a minimal number of plates. If precision pipeters are available a student can do a 10-4 dilution in replicate on two plates and a 10-8 dilution on 4.
Application: Any exercise which requires the enumeration of living bacteria by plate count.
Connectivity: Growth exercises (Ex. 1, 3, 5, 11, 13, 16) and harvesting exercises (Ex. 9, 10).
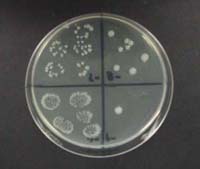
close description
| Exercise 8 - Flow Through Gram Stain description - instructor version - student version |
X | X |
|---|
Type: Grow, Vis
Description: Typical staining procedures require the slide to be heat fixed prior to staining. Since the biofilm matrix is > 95% water, heat fixing destroys precisely what one wishes to observe. In this Gram stain modification, the slide is never dried or heated. The biofilms are already "fixed" to the slide by their matrix.
Application: This technique can be used with any procedure that uses a microscope slide as the coupon (substratum).
Connectivity: Collection exercises (Exs. 6, 11, 16) and growth exercises (Exs. 1, 3, 13).
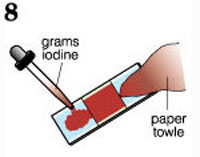
close description
| Exercise 9 - Harvesting and Dispersing of Cells from Biofilms (Standard Method) description - instructor version - student version |
X | X | X |
|---|
Type: Grow, Quant, Harv
Description: The two methods differ only in the method of dispersing the cells once they have been harvested. The standard method uses a Branson Ultrasonic Corporation or similar sonic water bath cleaner delivering approximately 50-60 hz, to disrupt biofilms and disperse cells, the alternative method uses a TurboMixTM High Performance Attachment ™ for the Vortex Genie mixer which can vortex 12 samples in microcentrifuge tubes simultaneously.
Application: This technique can be used with any protocol that harvests cells from a coupon such as a 1 x 3 slide or other flat surface.
Connectivity: This procedure will work well with Growth exercises such as (Exs. 1, 3 and 13), and with Collection techniques such as (Exs. 5 and 16).

close description
| Exercise 10 - Harvesting and Dispersing of Cells from Biofilms (Alternative Method) description - instructor version - student version |
X | X | X |
|---|
Type: Grow, Quant, Harv
Description: The two methods differ only in the method of dispersing the cells once they have been harvested. The standard method uses a Branson Ultrasonic Corporation or similar sonic water bath cleaner delivering approximately 50-60 hz, to disrupt biofilms and disperse cells, the alternative method uses a TurboMixTM High Performance Attachment ™ for the Vortex Genie mixer which can vortex 12 samples in microcentrifuge tubes simultaneously.
Application: This technique can be used with any protocol that harvests cells from a coupon such as a 1 x 3 slide or other flat surface.
Connectivity: This procedure will work well with Growth exercises such as (Exs. 1, 3 and 13), and with Collection techniques such as (Exs. 5 and 16).

close description
| Exercise 11 - Henrici's Microbial Fishing Technique description - instructor version - student version |
X | X | X | X |
|---|
Type: Con, Coll, Grow, Vis
Description: The point of this exercise is to collect mixed species biofilms from either natural water sources (streams, ponds, salt marshes etc) or laboratory or domestic sources (aquariums, rain barrels, traps in sinks (gross!) etc.).
Application: In this exercise students see real live mixed biofilms usually including protozoa and micro-invertebrates. These are some of the most diverse and fascinating biofilms one can find.
Connectivity: This is usually a stand alone exercise although after observing it alive for a while, one could follow up with the Flow through Gram Stain procedure, (Ex. 8.) to get some idea of bacterial diversity.
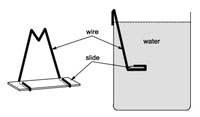
close description
| Exercise 12 - Measurement of Biofilm Thickness description - instructor version - student version |
X | X | X | X |
|---|
Type: Con, Grow, Vis, Quant
Description: This exercise uses a modified light microscope to measure the thickness of a biofilm prepared by any growth or collection method. The microscope is equipped with a pointer and a piece of polar coordinate graph paper so that by focusing on the surface and the base of the biofilm and recording the difference in degrees one can determine depth. Instructions for calibrating the microscope are included.
Application: This technique provides one more tool with which students can measure any biofilm on a flat transparent surface like a microscope slide.
Connectivity: This exercise can be used with any growth exercise (e.g. Exs. 1, 3, and 13) or any exercise in which biofilms are collected from nature (e.g. Exs. 5, 11, and 16).
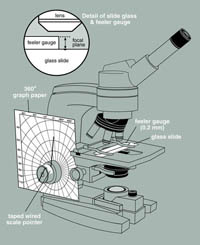
close description
| Exercise 13 - Model System for Growing a Standard Biofilm: Static Glass Coupon Reactor description - instructor version - student version |
X | X | X | X | X |
|---|
Type: Con, Grow, Vis, Quant, Indust
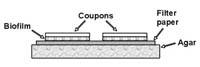
Description: This protocol was developed by scientists as S.C. Johnson to provide reproducible biofilms for testing disinfectant and cleansing products. A culture is established as a sort of sandwich growing between a filter paper and a microscope slide. When the slide is lifted from the filter paper, the biofilm, which adheres to the slide can be used in a variety of ways.
Application: The slide formed here can be used to visualize the biofilm, or to quantify the biofilm.
Connectivity: See Exs. 7, 8, 9, 10 and 12
close description
| Exercise 14 - Biofilms as Biobarriers description - instructor version - student version |
X | X | X | X |
|---|
Type: Con, Quant, Appl, Env, Indust
Description: In this exercise students construct a column containing sand or small glass beads. The column is charged with a culture of a nonpathogen that produces a great deal of EPS. The flow rate of liquid thorough the column is measured at intervals as the biobarrier forms.
Application: This technique has been used to slow the rate of movement of toxic chemicals in ground water to provide time for effective cleanup of spills.
Connectivity: This is a stand alone exercise.

close description
| Exercise 15 - Dental Biofilms: Detection and Quantification of Plaque description - instructor version - student version |
X | X | X |
|---|
Type: Vis, Quant, Class
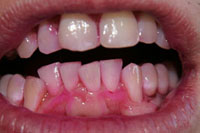
Description: In this exercise, students use a dental disclosing agent to show the extent of plaque attached to their teeth. A guide is given which aids students in quantifying the amount of plaque present.
Application: This exercise when combined with tooth brushing shows students the importance and effectiveness of brushing and of proper dental hygiene.
Connectivity: This exercise has been used with very young students (5th grade) on up to high-school and college students. Teens tend to be embarrassed by this exercise. Use your knowledge of your class to determine if this exercise is appropriate. With the cooperation of your local dental association you might couple this exercise with the distribution of new toothbrushes and information on dental care.
close description
| Exercise 16 - Isolation of Biofilm Populations from Soil Crumbs by Flotation description - instructor version - student version |
X | X | X | X | X | X |
|---|
Type: Con, Coll, Vis, Quant, Harv, Env
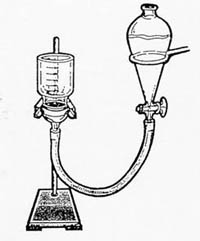
Description: The students construct a simple apparatus which permits them to harvest some biofilm associated cells from a crumb of soil by floatation on water.
Application: The population of cells isolated in this exercise can be stained for visualization, quantified, or cultured. A useful method of acquiring a population of cells that were associated with one another due to proximity in a single fragment of soil.
Connectivity: This exercise can be coordinated with Exs. 6, 7, 8, 9, 10, 20 and 22 (under development).
close description
| Exercise 17 - Bacterial Infection in a Cystic Fibrosis Patient description - instructor version - student version |
X | X |
|---|
Type: Class, Clinic
Description: This classroom exercises introduces students to the reality of Pseudomonas infections in Cystic fibrosis patients. The students are given some information from which they must draw inferences and conclusions. Then additional information is given permitting them to see more of the picture. The exercise, written by a CF medical specialists takes small portions of a couple of classes.
Application: Classroom interactive exercise.
Connectivity: This is a stand-alone exercise
close description
| Exercise 18 - Buccal Epithelial Cells with Adherent Bacterial Cells Revealed by Negative Staining description - instructor version - student version |
X | X | X | X |
|---|
Type: Coll, Vis, Quant, Clin
Description: Students harvest squamous epithelial cells from their cheek with a tongue depressor and stain the cells with nigrosine dye. This dye reveals the biofilm cells growing on the surface of the epithelial cells.
Application: This exercise gives students access to the most readily available and visible biofilm of the human body. These cells line the oral cavity and microbial populations extend into the epidermis as much a 3-4 cell layers deep. The cells are constantly being desquamated and replaced from below so the surface layers will have the greatest density of bacterial cells.
Connectivity: This is typically a stand alone exercise.

close description
| Exercise 19 - Construction of a Winogradsky Column description - instructor version - student version |
X | X | X | X |
|---|
Type: Con, Grow, Vis, Env
Description: In this exercise, students construct a miniature pond similar to those first built by Sergei Winogradsky. Given an appropriate container, some mud or soil, and samples of a carbon, nitrogen and sulfur source students can make columns which in time may become truly spectacular as highly pigmented photosynthetic microorganisms grow as a biofilm at the mud glass interface.
Application: This exercise provides a wonderful look at biofilms. Students can vary the starting nutritional and illumination conditions to produce different results.
Connectivity: One could isolate and quantify the organisms growing in the biofilm at the mud glass interface, the mud itself or in the water column above the mud layer. These could be cultured and quantified by many of the techniques given in this collection.

close description
| Exercise 20 - Construction of a Drip Flow Reactor description - instructor version - student version |
X | X | X |
|---|
Type: Con, Grow, Env
Description: Commercial Drip Flow reactors are quite expensive, this one can be made from materials in the lab or purchased at the local grocery or hardware store. A drip flow reactor is a type of Plug Flow reactor, which mimics the common habitat in nature in which water flows over a surface carrying nutrients which feed the biofilm growing on the surface. Rock walls, shower curtains, sinks and toilet bowls above the water line, are all examples of this sort of natural biofilm producing habitat.
Application: This type of reactor can be used to model many natural habitats in which the growing biofilm is "fed" by a continuous or intermittent flow of water.
Connectivity: The biofilms formed by a Drip Flow reactor can be used in the Flow through Gram stain exercise, or they can be quantified by harvesting and plating (Exs. 7, 9, 10). Thickness can be measured as in Ex. 12.

close description
| Exercise 21 - Coaggregation: Interactions between Bacteria in Dental Biofilms description - instructor version - student version |
|---|
Type: Con, Grow, Env
Description:
Application:
Connectivity:
close description
| Exercise 22 - Antimicrobial Sensitivity: Biofilm vs. Planktonic Cells (MBEC) description - instructor version - student version |
X | X | X | X |
|---|
Type: Con, Grow, Vis, Env
Description:
Application:
Connectivity:

close description
| Exercise 23 - Detection of Naturally Occurring Acyl-Homoserine Lactones Using a Chromobacterium Reporter Strain description - instructor version - student version |
|---|
Type: Con, Grow, Vis, Env
Description:
Application:
Connectivity:

close description
