Multicellularity
For those microbiologists who grew up and were trained in the paradigm that bacteria are “simple beasts” living singly in splendid isolation from one another and whose growth is controlled entirely by external factors such as temperature, pH, solute concentrations and nutrient availability, the concept of biofilms as complex interactive communities of multiple species comes as a shock. Of course we expect this sort of behavior from “real” multicellular eukaryotic organisms where disparate cell types with specialized roles, function under the integrated control of a nervous system, an endocrine system and a common genome. We were even aware of the few odd ball organisms like Myxococcus species, which behave in the very unbacterium-like fashion of swarming in a coordinated fashion and producing multicellular upright structures consisting of certain cells forming stalk while others specialize as spores.
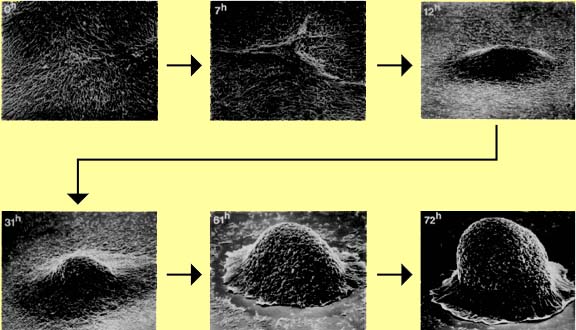
Figure 1. Formation of the fruiting body in Myxococcus xanthus.
We are quite unprepared, however, to give multicellular organism status to a cluster of bacteria no matter how organized they appear to be. Just how good is the evidence for multicellularity among biofilms?
Branda and Kolter (p 20 in Ghannoum and O’Toole) have written that“ Multicellularity is distinguished by a form of cell-cell cooperation in which the individual cells make investments in the group, in some cases sacrificing their ability to reproduce.” And they continue, “from an evolutionary perspective, this means that for maintenance of multicellularity the group of cells must be the unit of selection.”
The critical characteristics then appear to be, 1) intercellular communication, 2) coordination of cellular activities and 3) a division of labor even to the extent of certain cells giving up their ability to reproduce for the overall welfare of the community. If we accept this definition, do bacterial biofilms qualify as multicellular organisms?
- Intercellular communication both within and between species in biofilms is now a well-established phenomenon. Many bacteria have been shown to respond to soluble signaling molecules such as acyl-homoserine lactones (Gram negatives) and oligopeptides (Gram positives) in a great variety of cellular processes. Fruiting body formation in Myxococcus xanthus, for example, is under the control of signaling molecules called the A signal (Adventurous or Swarming motility). When nutrient runs low for M. xanthus, swarming cells begin to product the A signal and when the concentration of this signal reaches threshold levels aggregation of the swarm cells begins leading to the formation of a fruiting body with some cells producing non-reproductive stalk cells and others differentiating as spores.
- Coordination of cellular activities. The phenomenon of quorum sensing in bacteria is a control mechanism, which enables cells to delay certain activities until a sufficient population of cells is available for the delayed function to be expressed fully. In some instances the value of this process is not clear but in others is seems to make abundant "evolutionary sense". The marine bacterium Vibrio fisheri can exist either as a free-living bacterium in the ocean or as a symbiotic partner in the light producing organs of a variety of marine vertebrates and invertebrates such as the eyelight fish Photoblepharon palpebratum, and the bobtail squid Sepiola atlantica. The Vibrio continuously produce the signal molecule N-3-oxo-hexanoyl homoserine lactone (3-oxo-C6-HSL the product of the gene Lux I). In the open ocean, this molecule rapidly diffuses away so that its internal concentration is always low. Under these conditions the organism produces no light whatsoever. When enclosed within the vertebrate or invertebrate light organ, however, the population density of the bacterium can increase to enormous levels (1-5 x 1010 cells/gram). At this cell density the acyl homoserine lactone accumulates above some threshold level. At this point the molecule binds to a specific RNA polymerase, the product of the Lux R gene, which transcribes the Lux operon resulting in the production of the enzyme luciferase, which in turn oxidizes a long chain aldehyde producing light. What is the advantage of producing light at high, but not at low cell density? The induction of the Lux operon and the production of light is an energetically expensive process on the part of the bacterium. When encased in the nutrient rich light organ, the expenditure is energetically favorable and works to the advantage of both the bacterium and its host.

