Bacteria in a Biofilm Have Different Characteristics than the Same Bacteria in Isolation
Here is a somewhat startling characteristic of bacteria in a biofilm as observed by biofilm scientists and engineers. The same kind of bacteria are different when they are in a biofilm than when they are isolated in planktonic form (that is, floating as single cells in water). Let's think about this for a moment. This is one of those scientific discoveries that seems counterintuitive. It might seem so obvious that a bacteria cell is a bacteria cell is a bacteria cell that one might not even think to check whether a particular bacterium is different when it is found in different environments.
The details of how this is determined is an advanced topic, but you might find it interesting to hear how it is done.
The double-stranded helix structure of molecular DNA (deoxyribonucleic acid), discovered in 1953 (by Watson and Crick), has become a familiar image. The genes of all living organisms, composed of DNA, carry the “instructions” that determine the characteristics (phenotype) of the organism and are also the genetic material that is transmitted to the next generation by sexual or asexual reproduction
These genetic instructions function primarily through the synthesis of proteins. Some of these are structural components of the cell (flagellae for example), and many are enzymes the biological catalysts that carry out chemical reactions in the cell. These enzymes are responsible for energy production, acquiring nutrients from the surrounding environment, waste disposal and the manufacturer of the various components of which the cells are made. What may be surprising is that not all genes in the cell function all the time. There are to be sure certail “housekeeping” genes whose function is so essential that they function all the time, but other genes such as those used in spore production are in use only during certain points in the bacterial life cycle. So genes may be “upregulated” (turned on) or down regulated (turned off) as required by the cell. This ability to regulate gene function, like turning off a light switch as you leave a room, results in a significant savings in energy.
Interestingly, it has been discovered that within minutes of when a bacterium attaches to a surface it undergoes a remarkable change in gene function. Many operating genes (e.g. flagellae) are down regulated while others (slime production) are upregulated. How do we know?
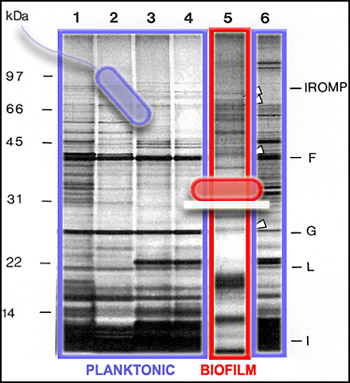
We can get a picture of cellular activity by examining the proteins produced by cells at a particular time. One way to get this kind of protein "snapshot" is by a technique called SDS-PAGE (for "Sodium Dodecyl Sulfate" and "PolyAcrylamide Gel Electrophoresis"). This technique allows scientists to see large (nearer the top) and small (nearer the bottom) cellular proteins as dark bands in an array of columns. In the SDS PAGE gel above, we see proteins from the outer membranes of planktonic (outlined in blue, Lanes 1-4 and 6) and biofilm (outlined in red, Lane 5) bacteria, of a single strain. Although we cannot tell from this gel just what these proteins are doing, we can see that the bands of proteins are strikingly different, telling us that the planktonic and biofilm forms of a single species are expressing different genes, and therefore carrying out different activities.
Estimates vary, but some suggest that as much as 40% of the genes of a bacterium may undergo up or down regulation in the transition from the planktonic to the biofilm state. So, even though the genes present haven’t changed at all the expression of those genes has undergone a dramatic shift, it is almost as if the two states (planktonic and biofilm) were entirely different organisms.
So what? Beyond the intellectual interest this holds for biofilm scientists and engineers, what practical use does this knowledge have? One example is in the development of antibiotics. These drugs traditionally have been developed to kill planktonic bacteria under the assumption that they would kill the same bacteria wherever they were found. We now know, however, that
- Planktonic bacteria are more susceptible to antimicrobial chemicals designed to kill them than are biofilm bacteria, and
- Many of the infections plaguing humans are actually caused by bacteria in the biofilm mode of growth, not the planktonic mode of growth.
Put these two things together with the fact that traditional antibiotics have been designed for and tested on bacterial cells in their relatively unprotected, planktonic state and we can begin to understand why it is that antibiotics don't work well on these same bacteria when they exist in a biofilm—the same bacterium is different in the biofilm state than in the planktonic state for which the antibiotic was designed and tested!
This presents scientists and engineers with a new challenge, namely the development of new classes of antibiotics that target bacteria that exist in the biofilm state. Understanding the genetic activity of biofilm bacteria will help us to find new ways to target these cells and disrupt their functions.


